ASPEN 学习笔记 8 :物性参数估算
前言
尽管ASPEN提供了足够的物性数据库,大部分情况下可能是够用了,但是谁知道呢,也许一不小心就遇到了某些物性数据缺失的情况,这种情况下就得对物性参数进行估算了。
纯组分的物性参数估算
估算噻唑 C3H3NS 的物性,查得其分子结构 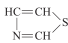 ,分子量85,正常沸点TB 116.8°C。我们假装不知道它的沸点,采用ASPEN来估算下。
,分子量85,正常沸点TB 116.8°C。我们假装不知道它的沸点,采用ASPEN来估算下。
进入Properties,选择Run mode为 Estimation,即估算
在Components的Specification 输入组分id,这里我输入化学式,其实系统能自动识别,说明其中应该是收录了这个物质的,这里不管这么多。
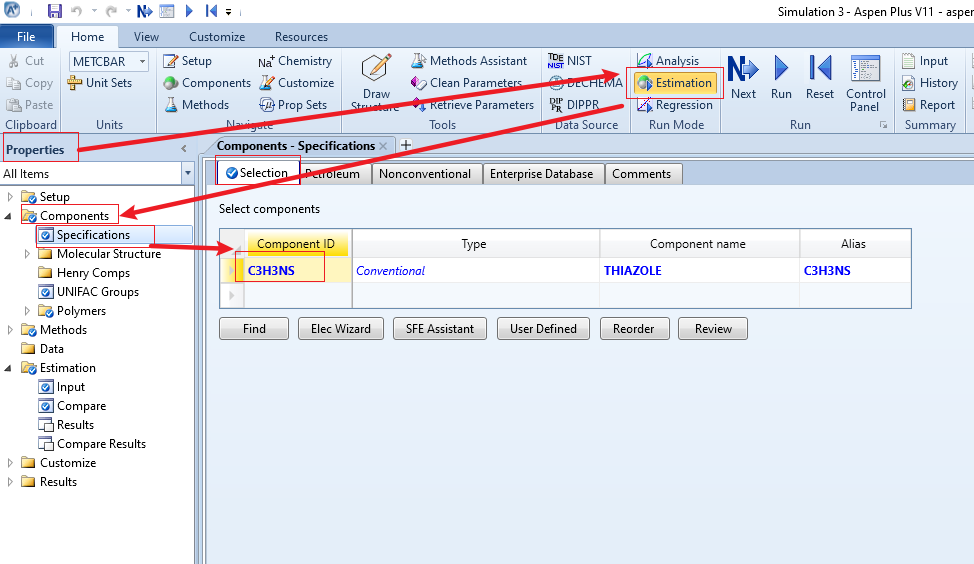
继续,这时发现已经没有未完成的项目提示,直接点Run试一下,可以运行,但是什么都没有。估计是因为没有指定要估算的物性。我们进入Estimation,input,Pure Components,Parameters 选择TB,同时指定组分为C3H3NS,选择估算方法,这里有四种我们都选择上,点击RUN试试看。

报错了

Processing input specifications ...
STRUCTURE FOR COMPONENT C3H3NS HAS NOT BEEN DEFINED.
PCES CANNOT USE GROUP-CONTRIBUTION METHODS TO ESTIMATE MISSING PROPERTIES
** ERROR IN PHYSICAL PROPERTY SYSTEM
PROP-NAME TB FOR COMPONENT C3H3NS CANNOT BE ESTIMATED USING
THE JOBACK METHOD AS SPECIFIED BECAUSE OF MISSING INPUT PARAMETERS:
STRUCTUR
** ERROR IN PHYSICAL PROPERTY SYSTEM
PROP-NAME TB FOR COMPONENT C3H3NS CANNOT BE ESTIMATED USING
THE OGATA-TS METHOD AS SPECIFIED BECAUSE OF MISSING INPUT PARAMETERS:
STRUCTUR
** ERROR IN PHYSICAL PROPERTY SYSTEM
PROP-NAME TB FOR COMPONENT C3H3NS CANNOT BE ESTIMATED USING
THE GANI METHOD AS SPECIFIED BECAUSE OF MISSING INPUT PARAMETERS:
STRUCTUR
** ERROR IN PHYSICAL PROPERTY SYSTEM
PROP-NAME TB FOR COMPONENT C3H3NS CANNOT BE ESTIMATED USING
THE MANI METHOD AS SPECIFIED BECAUSE OF MISSING INPUT PARAMETERS:
PL-DATA
! Errors while processing input specifications
从报错代码来看,前面三种需要结构数据,最后一种方法需要PL-DATA,这里把MANI方法取消
并进入Components -Molecular Structure 中
点击Calculate Bonds
再来Run一次

仍然报错,最后只选择JOBACK方法,估算得到TB为 387.84K,为114.69°C, 略有差距。
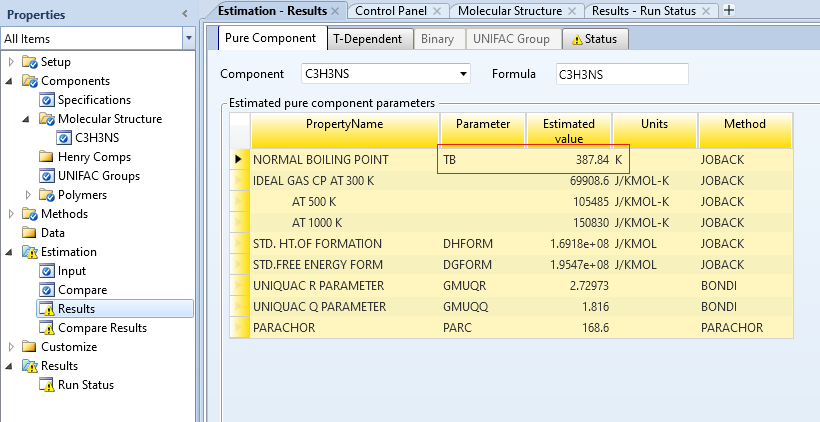
同时可以看到,在估算方法中,还有其他的方法,但是这里好像不适用,打开aspen help,查找boiling point
看到说明如下
| Method | Information Required |
|---|---|
| Joback | Structure |
| Ogata-Tsuchida | Structure |
| Gani | Structure |
| Mani | PC, Vapor pressure data (also uses TC if available) |
进一步查看
Table 3.5 Joback Method Functional Groups
Nonring Increments
| Functional Group | Group Number |
|---|---|
| -CH3 | 100 |
| >CH2 | 101 |
| >CH- | 102 |
| >C< | 103 |
| =CH2 | 104 |
| =CH- | 105 |
| =C< | 106 |
| =C= | 107 |
| ≡CH | 108 |
| ≡C- | 109 |
Ring Increments
| Functional Group | Group Number |
|---|---|
| >CH2 | 110 |
| >CH- | 111 |
| >C< | 112 |
| =CH- | 113 |
| =C< | 114 |
Halogen Increments
| Functional Group | Group Number |
|---|---|
| -F- | 115 |
| -CL | 116 |
| -BR | 117 |
| -I | 118 |
Oxygen Increments
| Functional Group | Group Number |
|---|---|
| -OH (alcohols) | 119 |
| -OH (phenols) | 120 |
| -O- (nonring) | 121 |
| -O- (ring) | 122 |
| >C=O (nonring) | 123 |
| >C=O (ring) | 124 |
| O=CH- (aldehyde) | 125 |
| -COOH (acid) | 126 |
| -COO- (ester) | 127 |
| =O (except as above) | 128 |
Nitrogen Increments
| Functional Group | Group Number |
|---|---|
| -NH2 | 129 |
| >NH (nonring) | 130 |
| >NH (ring) | 131 |
| >N- (nonring) | 132 |
| -CN | 133 |
| -NO2 | 134 |
| -N= (nonring) | 135 |
| -N= (ring) | 136 |
| =NH | 137 |
Sulfur Increments
| Functional Group | Group Number |
|---|---|
| -SH | 138 |
| -S- (nonring) | 139 |
| -S- (ring) | 140 |
Table 3.8 Ogata-Tsuchida Method Functional Groups
Refer to the table of Functional Group Abbreviations at the end of this chapter for details on some of the symbols used in this table.
Halogens
| Functional Group | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| RH | 100 | Me, t-Bu |
| RCL | 101 | |
| RBR | 102 | |
| RI | 103 |
Alcohols and Ethers
| Functional Group | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| ROH | 104 | Me, t-Bu |
| MeOR | 105 | Me |
| EtOR | 106 | |
| ROR | 107 | Me, Hep |
| PhOR | 108 | |
| RONO2 | 109 |
Sulfur Groups
| Functional Group | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| RSH | 110 | |
| RSMe | 111 | Me |
| RSET | 112 | |
| RSR | 113 | Me, Hep |
Amines and Nitro Compounds
| Functional Group | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| RNH2 | 114 | |
| RNHMe | 115 | |
| RNHEt | 116 | |
| RNHPr | 117 | |
| RNMe2 | 118 | Me |
| RNO2 | 119 | Me, Et |
Aldehydes and Ketones
| Functional Group | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| HCOR | 120 | |
| MeCOR | 121 | |
| EtCOR | 122 |
Cyanide
| Functional Group | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| RCN | 123 |
Acids and Esters
| Functional Group | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| RCOCL | 124 | |
| HCOOR | 125 | |
| MeCOOR | 126 | |
| EtCOOR | 127 | |
| PhCOOR | 128 | |
| RCOOH | 129 | |
| RCOOMe | 130 | |
| RCOOEt | 131 | |
| RCOOPr | 132 | |
| RCOOPh | 133 | |
| (RCO)2O | 134 | Hep |
| CLCH2COOR | 135 | |
| CL2CHCOOR | 136 | |
| BRCH2COOR | 137 | |
| NCCH2COOR | 138 | |
| CH2=CHCOOR | 139 |
Radicals
| Radical Type | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| METHYL | 140 | |
| ETHYL | 141 | |
| N-PROPYL | 142 | |
| ISOPROPYL | 143 | |
| N-BUTYL | 144 | |
| SEC-BUTYL | 145 | |
| ISOBUTYL | 146 | |
| T-BUTYL | 147 | |
| N-AMYL | 148 | |
| ISOAMYL | 149 | |
| T-AMYL | 150 | |
| NEOPENTYL | 151 | |
| N-HEXYL | 152 | |
| ISOHEXYL | 153 | |
| N-HEPTYL | 154 | |
| N-OCTYL | 155 | |
| VINYL | 156 | |
| ALLYL | 157 | |
| 2-BUTENYL | 158 | |
| PHENYL | 159 |
Molecules
| Molecule | Group Number | Radical, R, showing deviations > 5K |
|---|---|---|
| ETHANE | 160 | |
| PROPANE | 161 | |
| N-BUTANE | 162 | |
| ISOBUTANE | 163 | |
| N-PENTANE | 164 | |
| ISOPENTANE | 165 | |
| NEOPENTANE | 166 | |
| N-HEXANE | 167 | |
| ISOHEXANE | 168 | |
| N-HEPTANE | 169 | |
| N-OCTANE | 170 | |
| ETHYLENE | 171 | |
| PROPYLENE | 172 | |
| 2-BUTENE | 173 | |
| BENZENE | 174 |
Table 3.4A Gani Method Functional Groups
Refer to the table of Functional Group Abbreviations at the end of this chapter for details on some of the symbols used in this table.
The First-Order Groups
Nonring Increments
| Functional Group | Group Number |
|---|---|
| -CH3 | 1015 |
| >CH2 | 1010 |
| >CH- | 1005 |
| >C< | 1000 |
| -CH=CH2 | 1070 |
| -CH=CH- | 1065 |
| >C=CH2 | 1060 |
| -CH=C< | 1055 |
| >C=C< | 1050 |
| CH2=C=CH | 4995 |
| -C#CH (alkine) | 2655 |
| -C#C (alkine) | 2650 |
Benzene Ring Increments
| Functional Group | Group Number |
|---|---|
| -ACH= | 1105 |
| >AC= | 1100 |
| CH3-AC | 1160 |
| -CH2-AC | 1155 |
| >CH-AC | 1150 |
Oxygen Increments
| Functional Group | Group Number |
|---|---|
| -OH (alcohol) | 1200 |
| HO-AC (phenol) | 1350 |
| CH3-CO-(C) | 1405 |
| -CH2-CO-(C) | 1400 |
| O=CH- (aldehyde) | 1450 |
| CH3-COO-(C) (ester) | 1505 |
| -CH2-COO-(C) (ester) | 1500 |
| HCOO-(C) (formate) | 1550 |
| CH3-O-(C) (nonring) | 1615 |
| -CH2-O-(C) (nonring) | 1610 |
| >CH-O-(C) (nonring) | 1605 |
| -CH2-O-(C) (ring) | 1600 |
| -COOH (acid) | 1955 |
| -COO- (ester) | 3300 |
| -OC2H3OH- | 3605 |
| -O(CH2)2OH | 3600 |
Nitrogen Increments
| Functional Group | Group Number |
|---|---|
| -CH2-NH2 | 1655 |
| >CH-NH2 | 1650 |
| CH3-NH- | 1710 |
| -CH2-NH- | 1705 |
| >CH-NH- | 1700 |
| CH3-N< | 1755 |
| -CH2-N< | 1750 |
| NH2-AC (benzene ring) | 1800 |
| C5H4N- (pyridine ring) | 1855 |
| C5H3N< (pyridine ring) | 1850 |
| -CH2-C#N (nitrile) | 1900 |
| -CH2-NO2 | 2255 |
| >CH-NO2 | 2250 |
| NO2-AC (benzene ring) | 2300 |
| -CONH2 | 3550 |
| -CONHCH3 | 3555 |
| -CONHCH2- | 3560 |
| -CON(CH3)2 | 3565 |
| -CONCH3CH2- | 3570 |
| -CON(CH2)2< | 3575 |
| HCON(CH2)2 | 4996 |
Halogen Increments
| Functional Group | Group Number |
|---|---|
| -CH2-CL | 2010 |
| >CH-CL | 2005 |
| ->C-CL | 2000 |
| -CH<CL2 | 2055 |
| >C-CL2 | 2050 |
| -CCL3 | 2100 |
| CL-AC (benzene ring) | 2200 |
| -I | 2550 |
| -BR | 2600 |
| CL-(C=C) | 2800 |
| F-AC (benzene ring) | 2850 |
| -CF3 | 2960 |
| >CF2 | 2955 |
| >C<F | 2950 |
| -CCL2F | 3505 |
| -HCCLF | 3515 |
| -CCLF2 | 3520 |
| -F (except as above) | 3535 |
Sulfur Increments
| Functional Group | Group Number |
|---|---|
| -CH2-SH | 2400 |
| CH3S- | 3650 |
| -CH2S- | 3655 |
| >CHS- | 3660 |
| -C4H3S | 3755 |
| >C4H2S | 3760 |
Second-Order Groups Corrections
Nonring Corrections
| Functional Group | Group Number |
|---|---|
| (CH3)2CH- | 5000 |
| (CH3)3C- | 5005 |
| -CH(CH3)CH(CH3)- | 5010 |
| -CH(CH3)C(CH3)< | 5015 |
| -C(CH3)2C(CH3)2- | 5020 |
| CH3CH3 | 5050 |
| >C=C-C=C< | 5090 |
| -CH=C-C=C< | 5095 |
| CH2=C-C=C | 5100 |
| C=CH-C=C | 5105 |
| CH=CH-C=C | 5110 |
| CH2=CH-C=C | 5115 |
| CH=C-C=CH | 5120 |
| CH=C-C=CH2 | 5125 |
| CH2=C-C=CH2 | 5130 |
| CH2=CH-C=CH2 | 5135 |
| CH2=CH-C=CH | 5140 |
| CH2=CH-CH=CH2 | 5145 |
| CH3-C=C | 5150 |
| CH3-CH=C | 5155 |
| CH3-CH=CH | 5160 |
| CH3-CH=CH2 | 5165 |
| CH3-C=CH | 5170 |
| CH3-C=CH2 | 5175 |
| CH2-C=C | 5180 |
| CH2-CH=C | 5185 |
| CH2-CH=CH | 5190 |
| CH2-CH=CH2 | 5195 |
| CH2-C=CH | 5200 |
| CH2-C=CH2 | 5205 |
| CH-C=C | 5210 |
| CH-CH=C | 5215 |
| CH-CH=CH | 5220 |
| CH-CH=CH2 | 5225 |
| CH-C=CH | 5230 |
| CH-C=CH2 | 5235 |
| C-C=C | 5240 |
| C-CH=C | 5245 |
| C-C=CH | 5250 |
| C-C=CH2 | 5255 |
| C-CH=CH | 5260 |
| C-CH=CH2 | 5265 |
| c-C-CMH2:(M>1) | 5310 |
Ring Corrections
| Functional Group | Group Number |
|---|---|
| 3-Member | 5025 |
| 4-Member | 5030 |
| 5-Member | 5035 |
| 6-Member | 5040 |
| 7-Member | 5045 |
Oxygen Corrections
| Functional Group | Group Number |
|---|---|
| CHCHO | 5055 |
| CCHO | 5060 |
| CH3COCH2 | 5065 |
| CH3COCH | 5070 |
| CH3COC | 5075 |
| c-C=O | 5080 |
| ACCHO (benzene ring) | 5085 |
| CHCOOH | 5270 |
| CCOOH | 5275 |
| ACCOOH (benzene ring) | 5280 |
| CH3COOCH | 5285 |
| CH3COOC<- | 5290 |
| COCH2COO- | 5295 |
| COCHCOO | 5300 |
| COCCOO | 5305 |
| CO-O-CO | 5315 |
| ACCOO (benzene ring) | 5320 |
| CHOH | 5325 |
| COH | 5330 |
| C(OH)C(OH) | 5335 |
| CH(OH)C(OH) | 5340 |
| CH2(OH)C(OH) | 5345 |
| CH(OH)CH(OH) | 5350 |
| CH2(OH)CH(OH) | 5355 |
| CH2(OH)CH2(OH) | 5360 |
| c-COH | 5365 |
| c-CHOH | 5370 |
| C-O-C=C | 5490 |
| CH-O-C=C | 5495 |
| CH2-O-C=C | 5500 |
| C-O-CH=C | 5505 |
| C-O-C=CH | 5510 |
| C-O-C=CH2 | 5515 |
| CH-O-CH=CH | 5520 |
| CH-O-CH=CH2 | 5525 |
| CH-O-C=CH | 5530 |
| CH-O-C=CH2 | 5535 |
| CH2-O-C=C | 5540 |
| CH2-O-CH=C | 5545 |
| CH2-O-CH=CH | 5550 |
| CH2-O-CH=CH2 | 5555 |
| AC-O-C (benzene ring) | 5560 |
| AC-O-CH (benzene ring) | 5565 |
| AC-O-CH2 (benzene ring) | 5570 |
| AC-O-CH3 (benzene ring) | 5575 |
Nitrogen Corrections
| Functional Group | Group Number |
|---|---|
| C(OH)CN | 5375 |
| CH(OH)-CN | 5380 |
| CH(OH)-CNH | 5385 |
| CH2(OH)-CN | 5390 |
| CH(OH)-CNH | 5395 |
| CH(OH)-CNH2 | 5400 |
| CH2(OH)-CNH | 5405 |
| CH2(OH)-CHNH2 | 5410 |
| CH(OH)-CHNH | 5415 |
| CH(OH)-CH2NH2 | 5420 |
| CH2(OH)-CHNH | 5425 |
| CH2(OH)-CH2NH2 | 5430 |
| C(NH2)-C(NH2) | 5435 |
| CH(NH2)-C(NH2) | 5440 |
| CH2(NH2)-C(NH2) | 5445 |
| CH(NH2)-CH(NH2) | 5450 |
| CH(NH2)-CH2(NH2) | 5455 |
| CH2(NH2)-CH2(NH2) | 5460 |
| c-C-N-c-C | 5465 |
| c-CH-N-c-C | 5470 |
| c-CH-N-c-CH | 5475 |
| c-CH-NH-c-C | 5480 |
| c-CH-NH-c-CH | 5485 |
| C(NH2)-COOH | 5705 |
| CH(NH2)-COOH | 5710 |
| CH2(NH2)-COOH | 5715 |
Sulfur Corrections
| Functional Group | Group Number |
|---|---|
| c-C-S-c-C | 5580 |
| c-CH-S-c-C | 5585 |
| c-CH2-S-c-C | 5590 |
| c-CH2-S-c-CH | 5595 |
| c-CH2-S-c-CH2 | 5600 |
Halogen Corrections
| Functional Group | Group Number |
|---|---|
| C=CF | 5605 |
| CH=CF | 5610 |
| CH2=CF | 5615 |
| C=CHF | 5620 |
| CH=CHF | 5625 |
| CH2=CHF | 5630 |
| C=CBr | 5635 |
| CH=CBr | 5640 |
| CH2=CBr | 5645 |
| C=CHBr | 5650 |
| CH=CHBr | 5655 |
| CH2=CHBr | 5660 |
| C=CI | 5665 |
| CH=CI | 5670 |
| CH2=CI | 5675 |
| C=CHI | 5680 |
| CH=CHI | 5685 |
| CH2=CHI | 5690 |
| ACBr (benzene ring) | 5695 |
| ACI (benzene ring) | 5700 |
Mani Method
The Mani method was developed by Juan-Carlos Mani of Aspen Technology. This method estimates TB from the Riedel vapor pressure equation when one or two experimental temperature-vapor pressure data pairs are available. Such data is usually available for new specialty chemicals, especially for large molecules. This method can also be used to estimate TC and vapor pressure.
This method provides very accurate and reliable estimates of TB, TC and vapor pressure curve when some experimental vapor pressure data is available. It is very useful for complex compounds that decompose at temperatures below the normal boiling points.
The Riedel equation gives vapor pressure as a function of TB, TC and PC of the component. If one T-P pair is available, and TC and PC are known or estimated, the equation can be used to provide estimates of TB and vapor pressure. When two T-P pairs are available and PC is known or estimated, the equation can provide estimates of TB, TC, and vapor pressure.
尝试二
采用葡萄糖的分子式进行估算,实际葡萄糖的沸点约527°C
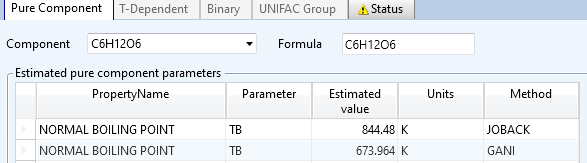
尝试三
采用乙醇分子式,实际乙醇沸点约78°C
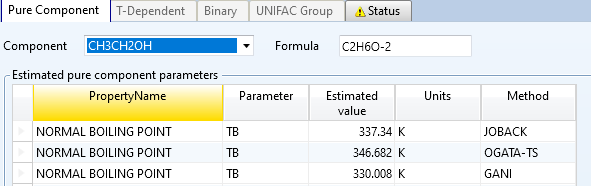
总结
mani 的方法是精确而可靠的,但是需要实验数据
其余根据结构来预测性质的,多少会有点偏差,要根据分子结构中含有的官能团选择合适的预测模型